
:max_bytes(150000):strip_icc()/nickelsulfate-56a128783df78cf77267ebb6.jpg)
Yield chemistry calculator how to#
So, how to find the actual yield? The calculation is simple if you know the percent and theoretical yields.Īll you need to do is plug the values into the calculator again: What is the actual yield of magnesium oxide?
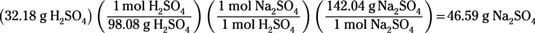
The theoretical yield is known to be 19 grams. For example, the decomposition of magnesium carbonate to form magnesium oxide has an experimental percent yield of 79 %. Reactants are converted to products, and the process is symbolized by a chemical equation. You can solve this problem by using our actual yield calculator:Įxample 2: When making a new substance from other substances, chemists say that they have synthesized a new compound. Let's move on to real-world applications now that we've learned about the distinctions between actual and theoretical yields.Įxample 1: If the percent yield of a reaction was 45 % with a theoretical yield of 4 g, what is the actual yield? Another reason for the increased yield is that the result is impure because another ingredient than the solvent is present.This happens most frequently when the solvent is still present in the product (incomplete drying), when the product is weighed incorrectly, or when an unaccounted ingredient in the reaction acts as a catalyst or causes the creation of by-products and.Sometimes your product may undergo unwanted reactions after you have formed it due to the presence of high temperatures or other chemicals.īut, it is also possible that the actual yield exceeds the theoretical yield:.Even if the substance is insoluble in a particular solvent, if you rinse it, a little bit of it may be lost due to dissolving in the solvent and.Some products may remain on the filter paper or make their way through the mesh and wash away if you filter the solution through filter paper.If you're recovering a precipitate, you can lose some of it if it doesn't completely crash out of the solution.The actual yield is usually lower than the theoretical yield because few reactions proceed to absolute completion (i.e., they aren't 100% efficient) or because not all of the product in a reaction is recovered.


 0 kommentar(er)
0 kommentar(er)
